Are You Ready For UDI Implementation?
DCG Technology Makes Compliance Easy
The U.S. FDA published its rule for Unique Device Identification (UDI) regulations in the United States. All Class III and Class II medical device manufacturers will be required to implement these new regulations. Our Delivery Systems Division is prepared to ensure that our customers understand their options and meet these new compliance rules effectively.
On the administration side, a UDI code must be legible for the life of the product. On the business side, they must be economical to produce. The cost and durability concerns associated with screen printing codes make this option out of the question. Laser marking codes are a viable option, but there are potential legibility issues, especially with repeated cleanings on reflective metal. Third-party labels may work, but only if a code data management structure is in place and the adhesive is guaranteed to hold.

This option applies a higher contrast ratio (legibility) as well as a more resilient finish (durability) than other code producing methods available. The DCG process
is produced completely in-house, making it very cost-effective. Because DCG is managed in-house, so will the data associated with generating UDI codes. The code information will never be subject to interpretation by a third-party vendor.
Considering these benefits, color choices, and traditional DCG features, PPM is pleased to have a solution for customers that allows them to fulfill UDI requirements while maintaining the aesthetics that set their brand apart.

|

Laser Marking Sample
Less color contrast. Over time and repeated cleaning, laser marking will degrade.
|
|
|
|

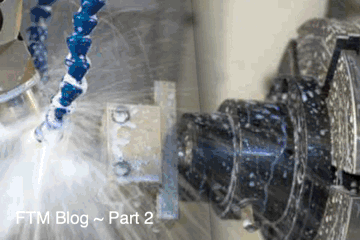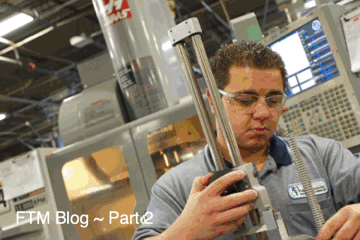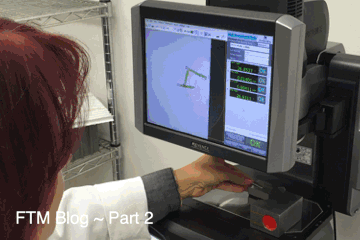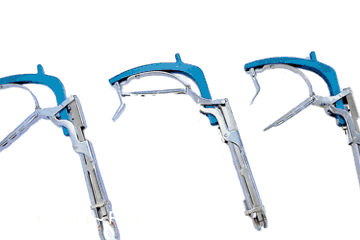
DCG UDI Sample
Delivers great color contrast and durability with DCG’s highly scratch-resistant finish.
|
|
|
|
Color and Aesthetics
DCG offers customers the option to use color while maintaining the same legibility and durability features.
|
|
|
|
|
|
If have questions about UDI implementation, call 201-797-8820 or email info@phillipsmedicraft.com.










Get Social